This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemists decipher reaction process that could improve lithium-sulfur batteries
Lithium-sulfur batteries can potentially store five to 10 times more energy than current state-of-the-art lithium-ion batteries at much lower cost. Current lithium-ion batteries use cobalt oxide as the cathode, an expensive mineral mined in ways that harm people and the environment. Lithium-sulfur batteries replace cobalt oxide with sulfur, which is abundant and cheap, costing less than one-hundredth the price of cobalt.
But there's a catch: Chemical reactions, particularly the sulfur reduction reaction, are very complex and poorly understood, and undesired side reactions could end the batteries' lives well before those of traditional batteries.
Now, researchers led by UCLA chemists Xiangfeng Duan and Philippe Sautet have deciphered the key pathways of this reaction. These findings, outlined in a paper published in the journal Nature, will help fine-tune the reaction to improve battery capacity and lifetime.
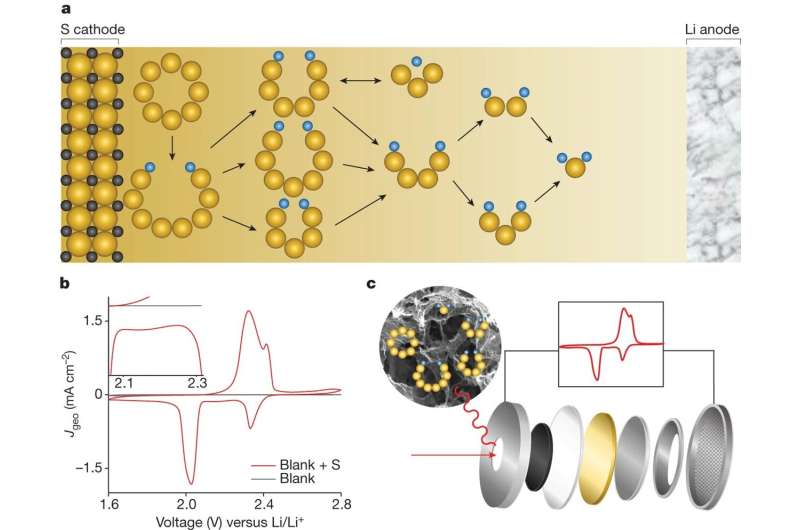
The sulfur reduction reaction in a lithium-sulfur battery involves 16 electrons to convert an eight-atom sulfur ring molecule into lithium sulfide in a catalytic reaction network with numerous interwoven branches and different intermediate products called lithium polysulfides and many other byproducts.
Because it is such a complex reaction, with many paths branching off from each other and many intermediate products that are important for continuing the reaction, it's been hard to study and even harder to figure out which parts of the reaction to target for improved battery performance.
"Despite extensive efforts devoted to improving the apparent performance of lithium-sulfur batteries, the fundamental reaction mechanism remains unsettled," said Duan, corresponding author and UCLA professor of chemistry and biochemistry. "The main branch in this reaction network for the sulfur reduction reaction remains a topic of considerable debate."
A problem of particular interest is a side reaction in which the polysulfide intermediates migrate, called shuttling, to the lithium metal anode and react with it, consuming both sulfur and lithium and leading to energy loss and rapidly reduced storage capacity. A clear identification of the key intermediates and better understanding of how these intermediates are produced or consumed would help scientists control this migration between electrodes and minimize the waste of sulfur and lithium.
The new study deciphers the whole reaction network for the first time, determines the dominant molecular pathway, and unveils the critical role of electrocatalysis in modifying the reaction's kinetics.
The team first used theory calculations to map out all possible reaction pathways and the associated intermediates and then electrochemical and spectroscopic analysis to validate the computational findings.
Battery performance was dominated by Li2S4 as the main intermediate, and catalysis turned out to be crucial for fully converting the Li2S4 to the final discharge product (Li2S). Carbon-based electrodes doped with sulfur and nitrogen can effectively facilitate this conversion.
Their study also found that the intermediate Li2S6 does not directly participate in the electrochemical process but is present as a significant product from side chemical reactions and contributes significantly to the unwanted polysulfide shuttling effect.
"Our study provides a fundamental understanding of the sulfur reduction reaction in lithium-sulfur batteries and demonstrates that a properly designed catalytic electrode material can accelerate the charging and discharging reactions, mitigate the side reactions, and improve the cycle life," said Duan.
"The combination of battery technology and catalysis science opens new avenues for fast and high-capacity energy conversion devices," said Sautet, who is the Levi James Knight, Jr. Term Chair for Excellence.
More information: Rongli Liu et al, Establishing reaction networks in the 16-electron sulfur reduction reaction, Nature (2024). DOI: 10.1038/s41586-023-06918-4