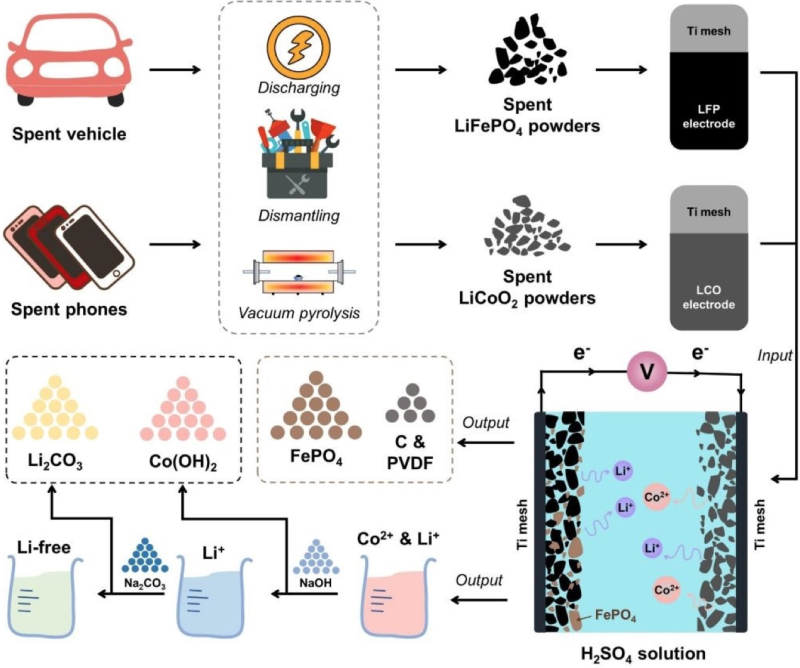
A team of Chinese researchers have presented a study proposing a paired electrolysis approach to recover spent lithium cobalt oxide (LiCoO2) and lithium iron phosphate (LiFePO4) electrode materials more efficiently. They published an open-access paper in the RSC journal Green Chemistry.
The researchers vacuum-pyrolyzed spent LCO and LFP cathodes to extract active components from aluminum foils. Working electrodes were made from powders coated on titanium mesh. The LCO and LFP dual-electrolysis experiments were performed in a low-sulfuric acid solution. Continuous voltage electrolysis used the LCO as the cathode and the LFP as the anode. In this process, LiCoO2 is reduced to release Co2+ and Li+ into the electrolyte and LiFePO4 is oxidized to FePO4 while releasing Li+ through a surface chemical reaction control process.
Through this approach, the leaching efficiencies of Li and Co reach above 98%. Since two electrode reactions simultaneously proceed in one electrolytic cell, the paired electrolysis has the benefit of maximizing energy efficiency, as well as reducing the amount of chemicals and secondary wastes, the researchers said.
Source: Green Car Congress