US Navy Prowls For E-Fuels From The Seven Seas
The e-fuels field has been stirring into life in recent years, thanks in part to the surging market for green hydrogen. The missing link is a ready supply of carbon dioxide. Various land-based systems under way to capture it from the air, but a team at MIT is onto a seagoing solution that dovetails with the needs of the maritime industry and the US Navy, too.
The US Navy Is Way Ahead On E-Fuels
The US Navy is always on the prowl for opportunities to resupply on-the-go. Back in 2009, for example, CleanTechnica took note of the Navy’s work on a high-efficiency on-board seawater desalination system.
In 2012, we noticed that the Navy was also working on seawater-to-fuel technology. We checked back again in 2015. Sure enough, the Navy had developed a system that simultaneously produces both hydrogen and captured carbon dioxide from seawater. Those are the indredients for liquid e-fuels. The Navy patented its hydrogen-plus-carbon device one year later, in 2016.
“With all the ingredients for making synthetic fuel at hand, the Navy anticipates being able to produce practically any kind of fuel it needs from seawater. Aside from JP-5 jet fuel, that includes LNG and CNG, as well as the multi-purpose fuel F-76,” we wrote.
Earlier this year, the U.S. Naval Institute also published a paper that explored the use of harvestable hydrogen for use in both seagoing and land-based expeditionary operations. They did not mean conventional hydrogen produced from natural gas. They meant green hydrogen pushed from water in an electrolysis system powered by wind, solar, or other renewable energy resources.
“Harvested fuel has numerous military advantages,” the authors observed. “Strategically, it reduces reliance on fuel imports and reduces the risks of supply chain disruptions. Harvesting hydrogen reduces the operational burden of transporting fuel and, tactically, reduces the visible logistics tail of friendly units.”
 Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
E-Fuels From The Seven Seas
The e-fuels field was slow to take off in the early years of the Obama administration. At that time the focus was biofuels and the leveraging of biological systems to produce renewable fuels. That began to change when the falling cost of renewable energy opened up the electrolysis pathway.
The growth of the offshore wind industry has added more fuel to the fire, so to speak. Offshore wind farms are beginning to piggyback with electrolyzer systems to produce green hydrogen at sea. The hydrogen can be piped to shore or loaded directly onto ships.
While the Navy is hammering away on its combination system, the flood of activity in the offshore green hydrogen production suggests an opportunity to produce e-fuels from seagoing carbon capture systems, and that’s where the new research from MIT comes in.
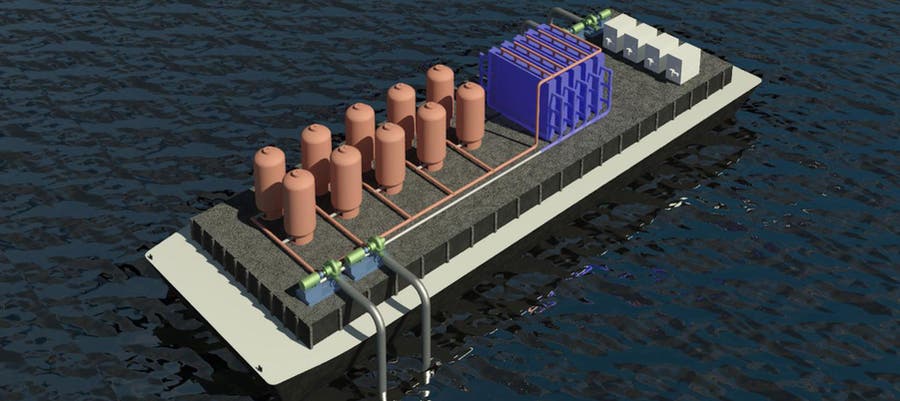
Last week, MIT News posted an article about the new system, described in a study by the team of professors T. Alan Hatton and Kripa Varanasi, postdoc Seoni Kim, and graduate students Michael Nitzsche, Simon Rufer, and Jack Lake, under the title, “Asymmetric chloride-mediated electrochemical process for CO2 removal from oceanwater.”
The study was published last week in the journal Energy & Environmental Science. “In recent years, the ocean has come to be recognized as a global-scale reservoir for atmospheric CO2,” the MIT team explains. “The removal of CO2 from oceanwater is thus considered a compelling approach to reduce ambient CO2 concentrations, and potentially achieve net-negative emissions.”
Electrolysis is one way to extract carbon dioxide from seawater. As MIT writer Chandler explains in the MIT News article, the conventional sequence involves converting bicarbonates to carbon dioxide, which is then vacuumed out.
The MIT team has proposed a more energy efficient, cyclical two-cell process that avoids expensive membranes and excess chemicals.
“… we report an asymmetric electrochemical system employing bismuth and silver electrodes that can capture and release chloride ions by Faradaic reactions upon application of appropriate cell voltages,” the team explains, adding that the system “can be leveraged for effective removal of CO2 from oceanwater without costly bipolar membranes.”
Acidified water from the MIT system is converted back to alkaline before being discharged to sea. The project received a $650,000 award from the Department of Energy’s cutting-edge energy technology funding office, ARPA-E, which observes that the “battery-like electro-swing approach does not require expensive membranes or addition of chemicals, is easy to deploy, and does not lead to formation of byproducts.”
“Innovative electrode configurations will be employed to reduce overall transport and electrical resistances while still enabling large quantities of water to be treated efficiently,” ARPA-E adds. “Relatively compact CO2 capture processes with promising low energetics, powered by renewable solar or wind resources, could be assembled for deployment on platforms or cargo ships.”
So…What About Direct Air Carbon Capture?
Fossil energy stakeholders have been touting direct air carbon capture as an effective climate action tool, but the MIT team points out that seawater already does the heavy lifting of carbon capture, by capturing the carbon. Ambient air carbon capture systems need to capture the carbon before anything else can take place.
“… the concentration of carbon dioxide in seawater is more than 100 times greater than it is in air,” Chandler notes. He cites Professor Hatton, who said that “the oceans are large carbon sinks…so the capture step has already kind of been done for you.”
The MIT team cautions that their system would have to reach an unwieldy scale on a global level in order to achieve a significant impact on ocean decarbonization overall. However, the system could be used to mitigate acid inputs at targeted sites, including fish farms and other aquaculture sites as well as offshore fossil energy operations.
The authors also make the case for adding their system to existing seawater desalination facilities, or attaching it to cargo ships.
Carbon, Carbon Everywhere
Don’t get too excited just yet. The MIT team is still tinkering with the system to improve efficiency. Overcoming mineral growth in the alkalinization cell is also a work in progress, but they expect to have a demonstration system up and running in about two years.
As of this writing, the team anticipates that their carbon capture system will easily outrun the production capacity of e-fuels stakeholders and other markets. They presume that a significant amount will have to be buried in underground formations.
However, by the time their system is ready for commercial operation, they may be pleasantly surprised to find that little, if any, captured carbon is being shunted underground.
In addition to the growing interest in e-fuels, the use of captured carbon in consumer goods is also gathering steam. The list of products made with recycled carbon includes plastic bottles, fleeces, perfumes, fabrics, eyewear, vodka, and more.
Local opposition to new carbon transportation and storage operations throws another obstacle in the path of carbon capture and sequestration.
Follow me on Trainwreck Twitter @TinaMCasey.
Find me on LinkedIn: @TinaMCasey or Mastodon: @Casey or Post: @tinamcasey
Image (cropped): System for capturing carbon dioxide from seawater courtesy of the researchers via MIT News.
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Latest CleanTechnica.TV Video

CleanTechnica uses affiliate links. See our policy here.