A research team led by the University of California San Diego has discovered the root cause of why lithium metal batteries fail. The team found that bits of lithium metal deposits break off from the surface of the anode during discharging and are trapped as “dead” or inactive lithium that the battery can no longer access.
The researchers say their discovery, which they recently described in the journal Nature, challenges the conventional belief that lithium metal batteries fail because of the growth of a layer called the solid electrolyte interphase (SEI), between the lithium anode and the electrolyte. The researchers made their discovery by developing a technique to measure the amounts of inactive lithium species on the anode and studying their micro- and nanostructures.
A major issue with lithium metal batteries is their low Coulombic efficiency. As the battery cycles, its stores of active lithium and electrolyte get depleted, so lithium metal batteries suffer from a limited number of cycles before they stop working. Battery researchers have long suspected that this is due to the growth of the SEI layer.
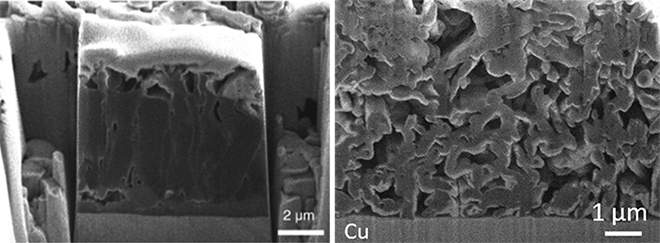

Although researchers have developed various ways to control and stabilize the SEI layer, they still have not fully resolved the problems with lithium metal batteries, explained senior author Y. Shirley Meng, a nanoengineering professor at UC San Diego.
“The cells still fail because a lot of inactive lithium is forming in these batteries. So there is another important aspect that is being overlooked,” Meng said.
The culprits, Meng and colleagues found, are lithium metal deposits that break off of the anode when the battery is discharging and then get trapped in the SEI layer. There, they lose their electrical connection to the anode, becoming inactive lithium that can no longer cycle through the battery. This trapped lithium is largely responsible for lowering the Coulombic efficiency of the cell.
The researchers identified the culprit by creating a method to measure how much unreacted lithium metal gets trapped as inactive lithium. In tests on lithium metal half-cells, researchers found that unreacted lithium metal is the main ingredient of inactive lithium. As more of it forms, the Coulombic efficiency sinks. Meanwhile, the amount of lithium ions from the SEI layer consistently stays low.
Moving forward, the team proposes strategies to control the depositing and stripping of lithium metal, such as applying pressure on the electrode stacks, creating SEI layers that are uniform and mechanically elastic, and using 3D current collectors.
“Control of the micro- and nanostructure is key,” Meng said. “We hope our insights will stimulate new research directions to bring rechargeable lithium metal batteries to the next level.”