Researchers at Tokyo Metropolitan University have developed a new method to make ceramic-based flexible electrolyte sheets for lithium metal batteries. They combined a garnet-type ceramic, a polymer binder, and an ionic liquid, producing a quasi-solid-state sheet electrolyte. The synthesis is carried out at room temperature, requiring significantly less energy than existing high-temperature (>1,000° C) processes. It functions over a wide range of temperatures, making it a promising electrolyte for batteries in EVs.
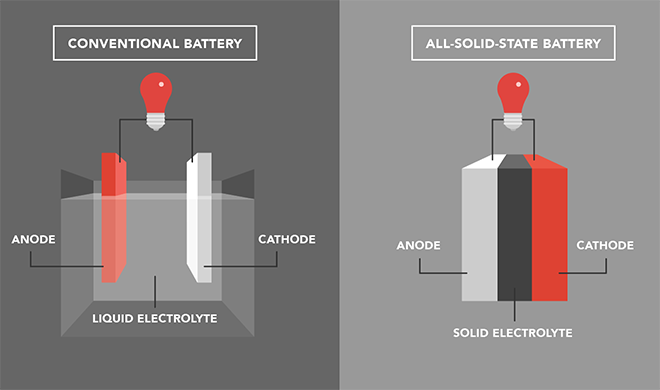
Lithium metal anodes have a much higher theoretical capacity than the graphite anodes currently in commercial use, but in liquid-based batteries, lithium dendrites can grow, possibly short-circuiting the battery and even leading to fires and explosions. Solid-state inorganic electrolytes are safer, and a garnet-type ceramic, Li7La3Zr2O12, better known as LLZO, is now widely regarded as a promising solid-state electrolyte material for its high ionic conductivity and compatibility with lithium metal. However, producing high-density LLZO electrolytes requires very high sintering temperatures, as high as 1,200° C. This is both energy-inefficient and time-consuming, making large-scale production of LLZO electrolytes difficult. In addition, the poor physical contact between brittle LLZO electrolytes and the electrode materials usually results in high interfacial resistance, greatly limiting their application in all-solid-state Li-metal batteries.
A team led by Professor Kiyoshi Kanamura at Tokyo Metropolitan University set out to develop a flexible composite LLZO sheet electrolyte which can be made at room temperature. They cast an LLZO ceramic slurry onto a thin polymer substrate, like spreading butter on toast. After drying in a vacuum oven, the 75-micron thick sheet electrolyte was soaked in an ionic liquid (IL) to improve its ionic conductivity. ILs are salts that are liquid at room temperature, known to be highly conductive while being almost non-flammable and non-volatile. Inside the sheets, the IL successfully filled the microscopic gaps in the structure and bridged the LLZO particles, forming an efficient pathway for lithium ions. They also effectively reduced interfacial resistance at the cathode. On further investigation, they found that Li-ions diffused through both the IL and the LLZO particles in the structure, highlighting the role played by both.
The synthesis is simple and suitable for industrial production, as the entire process is carried out at room temperature with no need for high-temperature sintering. Though challenges remain, the researchers say that the mechanical robustness and operability of the flexible composite sheet at a wide range of temperatures makes it a promising electrolyte for Li-metal batteries.
Source: Tokyo Metropolitan University